Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- One randomized control trial and 2 retrospective cohort studies found no significant differences between tenofovir and entecavir in the prophylaxis of hepatitis B virus reactivation in patients who were hepatitis B surface antigen positive and/or hepatitis B core antibody positive receiving chemotherapy or immunosuppressive therapy. There were no significant differences between these 2 drugs regarding renal function and other side effects.
- One randomized controlled trial found no patients in the tenofovir prophylaxis group had HBV reactivation compared to 10.7% in the observational group. However, the difference did not reach the level of statistical significance, probably owing to a small sample size. There were no significant differences between groups in terms of renal function, liver function, and other side effects.
- All 8 included guidelines strongly recommend the use of tenofovir or entecavir as antiviral prophylaxis in all patients with high risk of hepatitis B virus reactivation (hepatitis B surface antigen positive and/or hepatitis B core antibody positive) during chemotherapy or immunosuppressive therapy.
Context and Policy Issues
Hepatitis B virus (HBV) infection is a vaccine-preventable viral infection mainly affecting the liver that can cause both acute and chronic illness, causing permanent liver damage and liver cancer if left untreated.1 In 2018, there were a total of 4,783 cases in Canada, of which 189 cases were acute (0.52 per 100,000 population), 3,483 cases were chronic (10.6 per 100,000 population), and 751 cases were unspecified (2.1 per 100,000 population).1 Between 2009 and 2018, the overall rate of chronic HBV infection decreased from 13.4 to 10.6 per 100,000 population.1 There is no cure for the disease; however, it can be successfully treated with antiviral medications to slow down the disease progression and improve survival.1
Once the cells get infected by HBV, the covalently closed circular deoxyribose nucleic acid (DNA) remains permanently inside the host cells and serves as a template for future viral replication.2 Diagnosis and distinguishing between acute and chronic infections are made by serological testing to detect HBV surface antigen (HBsAg), HBV envelope antigen (HBeAg), HBV surface antibody (anti-HBs), HBV core antibody (anti-HBc), HBV envelop antibody (anti-HBe), and HBV-DNA. Acute HBV infection is defined when a person without a history HBV infection is first infected and loses the HBsAg within 6 months after the onset. Patients are diagnosed as having a chronic HBV infection if the HBsAg persist 6 months after onset of acute hepatitis.2 Patients whose HBV infections resolve within 6 months have undetected HBsAg and HBV-DNA but become anti-HBs-positive and anti-HBc-positive. People with immunity through vaccination have anti-HBs-positive, HBsAg-negative, anti-HBc-negative, and undetectable HBV-DNA.2
Cancer patients with chronic HBV (HBsAg-positive) or those with resolved or past HBV infection are at high risk for HBV reactivation during chemotherapy or immunosuppressive therapy.3,4 HBV reactivation is defined as an increase of serum HBV-DNA greater than 1 log10 IU/mL or a 10-fold increase from baseline, or a new HBV-DNA detection.5-7 When HBV reactivation presents with an increase in alanine aminotransferase (ALT) levels, there is an increased risk of mortality due to liver failure, interruption of cancer therapy and lower overall survival.4,8
There are 2 approaches of treatment of HBV reactivation, either by offering antiviral prophylaxis to all patients considered at moderate or high risk before starting chemotherapy or immunosuppressive therapy, or by regular monitoring of HBsAg, HBV DNA, and ALT, and starting antiviral therapy when HBV-DNA and/or ALT levels increase (so called pre-emptive approach).9 Antiviral prophylactic treatment has been found to be more effective in preventing HBV reactivation than the pre-emptive approach.10-12 A number of nucleoside and nucleotide analogue drugs, including lamivudine, telbivudine, clevudine, adefovir, entecavir, and tenofovir have been developed to block the HBV-DNA polymerase enzyme activity, thus inhibiting HBV replication.13 Of those drugs, entecavir and tenofovir have high antiviral potency and high genetic barrier making it less likely to develop drug-resistant HBV mutants during prolonged treatment.13
This report aims to summarize the clinical effectiveness of tenofovir for antiviral prophylaxis in patients with a history of HBV infection who are receiving oncology drug treatment. Additionally, this report aims to summarize the recommendations from evidence-based guidelines regarding the use tenofovir as antiviral prophylaxis for that patient population.
Research Questions
- What is the clinical effectiveness of antiviral prophylaxis with tenofovir for patients with history of hepatitis B who are receiving oncology drug treatment?
- What are the evidence-based guidelines for antiviral prophylaxis with tenofovir for patients with history of hepatitis B who are receiving oncology drug treatment?
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including MEDLINE, the Cochrane Database of Systematic Reviews, the International HTA Database, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy comprised controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were hepatitis B and tenofovir and cancer or cancer drugs. A CADTH-developed search filter was applied to limit retrieval to guidelines for a secondary search of the concepts hepatitis b and cancer or cancer drugs. The search was completed on July 19, 2022 and limited to English-language documents published since January 1, 2017.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, they were duplicate publications, they were published before 2017. Guidelines with unclear methodology were also excluded.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the following tools as a guide: the Downs and Black checklist14 for randomized and non-randomized studies, and the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument15 for guidelines. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 465 citations were identified in the literature search. Following screening of titles and abstracts, 431 citations were excluded and 34 potentially relevant reports from the electronic search were retrieved for full-text review. Eleven potentially relevant publications were retrieved from the grey literature search for full-text review. Of these 45 potentially relevant articles, 33 publications were excluded for various reasons, and 12 publications met the inclusion criteria and were included in this report. These comprised 2 randomized controlled trials (RCTs), 2 non-randomized studies, and 8 evidence-based guidelines. Appendix 1 presents the PRISMA16 flow chart of the study selection.
Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Additional details regarding the characteristics of included clinical studies (Table 2) and guidelines (Table 3) are provided in Appendix 2.
Study Design
The 4 included primary clinical studies comprised 2 RCTs17,18 and 2 retrospective cohort studies.19,20 The RCTs were published in 202117 and 2017,18 while the 2 retrospective cohort studies were published in 202119 and 2018.20 One RCT17 was parallel and from a single centre (a university). Blinding status in this RCT17 was not reported. The other RCT18 was multi-centre (17 hospitals), phase IV, open-label, and parallel. Both RCTs reported that sample size calculation for primary outcomes was performed. One RCT18 analyzed the data using the intention-to-treat (ITT) approach, while the other17 analyzed the data as per protocol. The 2 retrospective cohort studies19,20 did not perform sample size calculation and did not identify and adjust for confounding variables in the analyses.
All the included guidelines21-28 provide recommendations for the diagnosis, prophylaxis, and/or management of HBV infection. All included guidelines were not explicit about evidence collection, selection, and synthesis. Six guidelines.21,23-25,27,28 graded the level of evidence and the strength of recommendations using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.21,23-25,27,28 Two guidelines22,26 graded the level of evidence and the strength of recommendations using predefined criteria.
Country of Origin
The primary clinical studies were conducted by authors from Turkey,17 Spain,18 Japan,19 and Taiwan.20
The guidelines were conducted by authors from Australia,21,24 Germany,22 Brazil,23 India,25 US,26 Canada,27 and Italy.28
Patient Population
One RCT17 involved patients undergoing immunosuppressive treatments for onco-hematologic diseases, who had HBsAg and/or anti-HBc positivity, and were susceptible HBV reactivation. The other RCT18 involved patients with hematological malignancy receiving rituximab-based regimens either as monotherapy or in combination with chemotherapy. One retrospective cohort study19 involved patients undergoing chemotherapy or immunosuppressive therapy for cancer who had previous HBV infection or were HBV carriers. The other retrospective cohort study20 involved HBsAg-positive cancer patients undergoing chemotherapy.
The target population in the included guidelines21-28 were patients with HBV infection including those with high risk of HBV reactivation during chemotherapy or immunosuppressive treatments for hematological and solid tumour malignancies.
Interventions and Comparators
Two clinical studies17,20 compared tenofovir disoproxil fumarate (TDF) (245 or 300 mg/day) with entecavir (ETV) (0.5 mg/day), 1 study19 compared tenofovir alafenamide (TAF) (dosage not reported) with ETV (dosage not reported), and 1 study18 compared TDF (300 mg/day) with observation. The drugs were used as antiviral prophylaxis with a treatment period ranging from 24 weeks to 18 months.
All the included guidelines21-28 considered antiviral prophylaxis drugs, such as ETV, TDF or TAF, for patients undergoing chemotherapy or immunosuppressive treatments.
Outcomes
The outcomes considered in the included primary clinical studies were HBV reactivation including HBV reactivation rates17,18,20 and rates of undetectable HBV-DNA levels.17,19 The treatment-related side effects were kidney function,18-20 liver function,18 and others.17
All the included guidelines21-28 considered efficacy and safety outcomes related to the intervention and practice considered.
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included primary clinical studies (Table 4) and guidelines (Table 5) are provided in Appendix 3.
With respect to reporting, the 2 RCTs17,18 and 2 retrospective cohort studies19,20 clearly described the study objectives, interventions of interest, main outcome measures, and the main findings. The baseline patient characteristics were described in all studies. All studies reported treatment-related adverse events. Both RCTs17,18 did not describe the characteristics of patients lost to follow-up. One RCT18 used the ITT approach in the data analyses to account for patients lost to follow-up. Not accounting for patients lost to follow-up in the analyses may increase potential risk of attrition bias. In both retrospective cohort studies,19,20 there were no apparent group differences in most reported demographics of the included patients. However, there may exist confounding variables in both studies that were not identified and adjusted, and their impact on the findings is unknown. Actual P values (i.e., P values) and measures of random variability (e.g., confidence interval, standard deviation, or interquartile range) in the data for the main outcomes were reported in all included studies.17-20 Regarding external validity, it was unclear if the patients represent the entire population from which they were recruited in all included studies. For internal validity, 1 RCT17 did not report whether blinding to patients, investigators and outcome assessors was applied. The other RCT18 was an open-label trial. Non-blinding of patients and personnel may lead to performance bias, and non-blinding of outcome assessors may result in detection bias. Both RCTs17,18 did not report the methods of allocation concealment. Not performing allocation concealment may result in risk of selection bias. Sample size calculation was performed in both RCTs,17,18 but in 1 RCT,18 the calculated sample size was not reached, suggesting the study did not have the anticipated power to detect a clinically important effect. Both retrospective cohort studies19,20 may be prone to high risk of bias for selection, performance, and detection due to the nature of the observational study design. Additionally, confounding variables that could have significant impact on the findings were not identified and adjusted for in the analyses in these studies.19,20 Appropriate statistical tests were used to assess the main outcomes, and reliable and validated outcome measures were used in all included studies.17-20 Overall, all the included clinical studies17-20 were of low methodological quality.
All included guidelines21-28 were explicit in terms of scope and purpose (i.e., objectives, health questions and populations), and had clear presentation (i.e., specific, unambiguous, and easy to find key recommendations, with options for managing the different conditions or health issues). In terms of stakeholder involvement, all included guidelines21-28 clearly defined target users and the development groups. However, it was unclear if the views and preferences of the patients were sought in all guidelines except the Australian guideline.21 All the included guidelines21-28 did not clearly report on evidence collection, criteria for selection and on evidence synthesis. However, there were explicit link between recommendations and the supporting evidence, and methods of formulating the recommendations in all guidelines.21-28 Also, all guidelines21-28 considered health benefits and risks of side effects in formulating the recommendations. They included procedures for updating the guidelines and were externally peer-reviewed before publication. Two guidelines22,26 used the predefined criteria while 6 guidelines21,23-25,27,28 used the GRADE methodology to assess the level of evidence and grade their recommendations. It is unknown if the criteria had been validated for assessing the clinical guidelines. For applicability, it was unclear in terms of facilitators and barriers to application, advice and/or tools on how the recommendations can be put into practice, resource implications, and monitoring or auditing criteria in all included guidelines, except the Australian guideline by Doyle at al. (2019).24 For editorial independence, all guidelines21-28 reported competing interests of guideline development group members, but did not report if the views of the funding body had any influence on the content of the guidelines. Overall, all the included guidelines were of moderate methodological quality.
Summary of Findings
Appendix 4 presents the main study findings of the primary clinical studies17-20 (Table 6 and Table 7) and the summary of guideline recommendations21-28 (Table 8).
Clinical Effectiveness of Antiviral Prophylaxis With Tenofovir for Patients With History of Hepatitis B Who Are Receiving Oncology Drug Treatment
One RCT17 and 1 retrospective cohort study20 compared TDF with ETV, 1 retrospective cohort study19 compared TAF with ETV, and 1 RCT18 compared TDF with observation.
HBV Reactivation
Tenofovir Disoproxil Fumarate Versus Entecavir
The RCT by Toka et al. (2021)17 found that, in patients who were HBsAg and/or anti-HBc IgG positive and scheduled to receive immunosuppressive treatments for oncologic and hematologic diseases, all patients became HBV-DNA negative within 12 months of starting antiviral prophylaxis with TDF or ETV. Antiviral prophylaxis was given during immunosuppressive treatments. There was no significant difference in time to achieve DNA negativity in TDF group compared with ETV group (5.22 ± 3.02 months versus 5.40 ± 3.16 months; P = 0.84). There was no HBV reactivation in both groups, defined as at least 1 log increase in HBV-DNA from baseline levels or reappearance of HBV-DNA in individual who previously had an undetectable HBV-DNA. Patients were follow-up 1 year after completion of prophylaxis. During that the follow-up period, 14.3% of patients in the TDF group and 10.8% of patients in ETV group had HBV reactivation. A measure of statistical significance, such as P value, was not reported.
The retrospective cohort study by Lee et al. (2018)20 found that, for a median of 14 months treatment with TDF or ETV as antiviral prophylaxis in HBsAg-positive cancer patients undergoing chemotherapy, 95.5% and 85.7% of patients in the TDF and ETV groups, respectively, achieved undetectable HBV-DNA; P = 0.056. HBV reactivation rates, defined as an increase in HBV DNA levels 10-fold or more compared with the previous nadir levels, were 0.9% (1 patient) in the TDF group and 0.9% (1 patient) in the ETV group; P = 1.00.
Tenofovir Alafenamide Versus Entecavir
The RCT by Inada et al. (2021)19 found that, in patients receiving TAF or ETV as prophylaxis against or treatment for HBV reactivation, there was no significant difference between groups in the reduction in serum HBV-DNA from baseline to week 24 (−3.04 ± 2.47 versus −2.83 ± 1.45; P = 0.857). At week 24, HBV-DNA was undetectable in serum of 90.9% of patients in the TAF group versus 78.8% of patients in the ETV group (78.8%); P = 0.681.
Tenofovir Disoproxil Fumarate Versus Observation
The RCT by Buti et al. (2017)18 found that, in anti-HBc-positive patients with hematological malignancy receiving rituximab-based regimens either as monotherapy or as combination with chemotherapy, HBV reactivation (defined as HBsAg and/or HBV-DNA detection, or a confirmed ≥ 1 log IU/mL increase in HBV-DNA levels from baseline) was 0% in the TDF group compared to 10.7% in the observation groups after 18 months of treatment; P = 0.091. The results were similar in both intention-to-treat analysis and per protocol analysis.
Side Effects – Renal Function
Tenofovir Disoproxil Fumarate Versus Entecavir
The retrospective cohort study by Lee et al. (2018)20 found no significant difference in risk of renal events in HBsAg-positive cancer patients receiving TDF or ETV as antiviral prophylaxis during chemotherapy. Parameters investigated included incidence of acute kidney injury (33% versus 38.9%; P = 0.441), incidence of sustained kidney injury (11.3% versus 11.5%; P = 1.00), decrease in the estimated glomerular filtration rate (eGFR) of at least 20% (59.4% versus 60.2%); P = 1.00), decrease in eGFR ≥ 50% (9.4% versus 18.6%; P = 0.081), eGFR < 60 mL/min (27.4% versus 38.9%; P = 0.094), eGFR of less than 30 mL/min (3.8% versus 11.5%; P = 0.060), dose of TDF or ETV adjustment (12.3% versus 23.9%; P = 0.040), and serum phosphorous of less than 2 mg/dL (4.7% versus 4.4%; P = 1.000).
Tenofovir Alafenamide Versus Entecavir
The retrospective cohort study by Inada et al. (2021)19 found no significant difference in the decrease in the eGFR between the TAF group (−3.67 ± 13.19 mL/min/1.73 m2) and the ETV group (−0.62 ± 11.22 mL/min/1.73 m2); P = 0.291 at week 24.
Tenofovir Disoproxil Fumarate Versus Observation
The RCT by Buti et al. (2017)18 reported that the between-group analyses showed no significant differences between TDF and observation in renal function parameters, including serum creatinine, GFR, creatinine clearance, and serum phosphate at baseline and at month 18. The data for that comparison were not reported.
Side Effects – Liver Function
Tenofovir Disoproxil Fumarate Versus Observation
The RCT by Buti et al. (2017)18 reported that the between-group analyses showed no significant differences between TDF and observation in liver function parameters, including alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, bilirubin, albumin, alkaline phosphatase, and platelets at baseline and at month 18. The data for that comparison were not reported.
Side Effects – Others
Tenofovir Disoproxil Fumarate Versus Entecavir
The RCT by Toka et al. (2021)17 found no significant difference between TDF and ETV groups in the proportion of patients who had at least 1 side effects that did not require treatment disruption (23.3% versus 16.7%; P = 0.77). Examples of the side effects were sleep disturbances, headache, hematuria, abdominal pain, myalgia, nausea, weakness, and itching or rash on skin. One patient in the TDF group had to switch to ETV due to severe itchy, maculopapular, rash-like lesions. 17About 35% of patients in both groups died due to primary disease during prophylaxis, but the authors did not investigate whether antiviral prophylaxis with EVT or TDF played any role in the cause of deaths.
Guidelines Regarding the Use of Antiviral Prophylaxis With Tenofovir for Patients with History of Hepatitis B Who Are Receiving Oncology Drug Treatment
The Australian guideline21 published in 2022 strongly recommends entecavir or tenofovir treatment for HBsAg positive patients receiving cancer chemotherapy. The guideline also strongly recommends entecavir or tenofovir treatment for HBsAg-negative and anti-HBc positive patients who are being treated with drugs that are associated with a high risk of HBV reactivation.
Another Australian guideline24 published in 2019 also strongly recommends the use of entecavir or tenofovir as antiviral prophylaxis as soon as possible in all children or adult HBsAg positive patients with hematological or solid tumour malignancy undergoing higher risk cancer therapy (hematopoietic stem-cell transplantation [HSCT]; B-cell depleting, B-cell active or anti-CD20 drugs; acute leukemia and high-grade lymphoma therapy).
The German guideline22 published in 2021 strongly recommends the use of either tenofovir or entecavir in patients at high risk for HBV reactivation such as those with HBsAg- and/or anti-HBc positive undergoing high-dose chemotherapy, autologous stem-cell transplantation, steroid medication, and anti-CD20-antibodies treatment.
The Brazilian guideline23 published in 2020 strongly recommends that entecavir or tenofovir should be used as antiviral prophylaxis in all patients with high risk of HBV reactivation (i.e., HBsAg-positive and anti-HBc-positive, or HBsAg-negative and anti-HBc-positive) undergoing chemotherapy or immunosuppressive therapy. Treatment with entecavir or tenofovir should be maintained for 6 months, or 12 to 18 months in case of rituximab, after discontinuation of chemotherapy or immunosuppressive therapy.
The Indian guideline25 published in 2018 strongly recommends that entecavir or tenofovir should be used as antiviral prophylaxis to prevent HBV reactivation in adult patients undergoing chemotherapy or immunosuppressive therapy. Entecavir should be used for children 2 years and older; whereas entecavir or tenofovir can be used for children 12 years and older. The guideline also strongly recommends that antiviral prophylaxis therapy with entecavir or tenofovir should be started immediately in HBsAg-positive or HBV-DNA positive patients. In anti-HBc-positive, but HBsAg and HBV DNA negative patients, antiviral prophylaxis against HBV reactivation can be initiated in those at high-risk groups such as patients with lymphoma under a rituximab-containing regimens or those undergoing HSCT. The guideline strongly recommends that antiviral prophylaxis treatment should be continued for at least 12 months after discontinuation of chemotherapy or immunosuppressive therapy, or 18 months for rituximab-based regimens and HSCT.
The American guideline26 published in 2018 moderately recommends entecavir or tenofovir be used as antiviral prophylaxis in patients with high risk of HBV reactivation undergoing cancer therapy.
The Canadian guideline27 published in 2018 strongly recommends that patients undergoing immunosuppressive therapy or chemotherapy with high risk of HBV reactivation, such as those with HBsAg-positive, should undergo either monitoring or prophylactic therapy with entecavir or tenofovir. Monitoring or prophylactic therapy should also be applied to HBsAg-negative, anti-HBc-positive patients. The guideline strongly recommends that monitoring or prophylactic therapy should be continued for at least 12 months after completion of immunosuppressive therapy, or longer in patients who received B-cell depleting therapies.
The Italian guideline28 published in 2017 strongly recommends that antiviral prophylaxis drugs such as entecavir or tenofovir should be used to treat HBsAg-positive patients undergoing chemotherapy. The guideline also strongly recommends antiviral prophylaxis be initiated at least 1 week before or at the same time when starting chemotherapy. Antiviral prophylaxis should be administered during chemotherapy and should be continued for at least 12 to 24 months after completion of chemotherapy. The guideline also recommends subsequent monitoring for late HBV reactivation after the termination of the antiviral prophylaxis.
Limitations
The included clinical studies had several limitations. Both RCTs17,18 were limited in terms of sample size. Although sample size calculation was performed in the RCT by Toka et al. (2021),17 about 35% of patients died during prophylaxis therapy due to primary disease, leading to uncertainty about the study’s power to detect a significant difference between groups in the analysis. In the RCT by Buti et al. (2017),18 the calculated sample size was not reached; therefore, it was unclear whether the observed trend of numerically greater effectiveness of TDF than close monitoring in reducing HBV reactivation rate could reach statistical significance if the study had included the pre-estimated sample size. HBV genotype was not evaluated or reported in both RCTs.17,18 The non-randomized design of the 2 retrospective cohort studies19,20 indicate that they have risk of selection bias. The retrospective cohort study by Lee et al. (2018)20 enrolled patients with selected baseline characteristics, such as those with serum creatinine less than 1.2 mg/dL and those without liver cirrhosis, thus limiting the generalizability of the findings. The follow-up period in the retrospective cohort study by Inada et al. (2021)19 may not be long enough (i.e., 24 weeks) to examine the long-term safety and effects of TAF or ETV. The course of monitoring after TDF/TAF or ETV discontinuation was not described in any of the included studies.17-20
All the included guidelines21-28 did not clearly report the methods of collection, selection, and synthesis of the evidence.
Conclusions and Implications for Decision- or Policy-Making
This report identified 2 RCTs,17,18 2 retrospective cohort studies,19,20 and 8 guidelines.21-28 The identified primary clinical studies provided evidence for the efficacy and safety of tenofovir compared with entecavir,17,19,20 or tenofovir compared with observation,18 as antiviral prophylaxis against HBV reactivation in patients with history of HBV infection undergoing oncology drug treatment.
Efficacy against HBV reactivation was similar between tenofovir and entecavir, with no significant difference between the 2 for antiviral prophylaxis in patients who were HBsAg and/or anti-HBc positive receiving chemotherapy or immunosuppressive therapy. There was also no significant difference in renal function or other side effects between tenofovir and entecavir for antiviral prophylaxis. HBV reactivation did not occur in patients given tenofovir prophylaxis (0%) compared with observation group (10.7%), but the difference was not statistically significant. In terms of safety, patients in tenofovir group showed no significant differences in renal function and liver function parameters compared with those in the observation group.
All included guidelines21-28 strongly recommend the use of tenofovir or entecavir as antiviral prophylaxis in all patients with high risk of HBV reactivation (HBsAg-positive and/or anti-HBc-positive) during the course of chemotherapy or immunosuppressive therapy. Some guidelines23,25,28 recommend that antiviral prophylaxis with tenofovir or entecavir continues for 6 months to 18 months after completion of chemotherapy or immunosuppressive therapy. Thus, entecavir and tenofovir are favourable drugs in the prophylaxis and treatment of HBV reactivation.29
Given the unanimous recommendations of the included guidelines and the evidence from the included clinical studies, tenofovir and entecavir appear to be efficacious and safe as antiviral prophylaxis drugs to prevent HBV reactivation in patients undergoing chemotherapy or immunosuppressive therapy. The findings in this report are applicable to the Canadian context. One guideline25 from India recommends using entecavir in children 2 years and older, and entecavir or tenofovir in adults or children 12 years and older. One Australian guideline24 also recommends the use of entecavir or tenofovir in all children or adult HBsAg positive patients. However, the choice between tenofovir and entecavir for specific populations remains to be determined. Also, well-controlled trials with larger sample sizes and longer follow-up periods are needed.
Abbreviations
- ALT
alanine aminotransferase
- Anti-HBc
HBV core antibody
- Anti-HBe
HBV envelop antibody
- Anti-HBs
HBV surface antibody
- DNA
deoxyribose nucleic acid
- ETV
entecavir
- eGFR
estimated glomerular filtration rate
- HBeAg
HBV envelope antigen
- HBsAg
HBV surface antigen
- HBV
hepatitis B virus
- Ig
immunoglobulin
- ITT
intention-to-treat
- RCT
randomized controlled trial
- TAF
tenofovir alafenamide
- TDF
tenofovir disoproxil fumarate
References
- 1.
- Health Canada. Report on hepatitis B and C in Canada: 2018. 2021; https://www
.canada.ca /en/public-health/services /publications /diseases-conditions /report-hepatitis-b-c-canada-2018.html. Accessed 2022 Aug 8. - 2.
- Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319(17):1802-1813. [PubMed: 29715359]
- 3.
- Mindikoglu AL, Regev A, Schiff ER. Hepatitis B virus reactivation after cytotoxic chemotherapy: the disease and its prevention. Clin Gastroenterol Hepatol. 2006;4(9):1076-1081. [PubMed: 16861051]
- 4.
- Wang B, Mufti G, Agarwal K. Reactivation of hepatitis B virus infection in patients with hematologic disorders. Haematologica. 2019;104(3):435-443. [PMC free article: PMC6395346] [PubMed: 30733266]
- 5.
- Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61(2):703-711. [PMC free article: PMC5497492] [PubMed: 25412906]
- 6.
- Law MF, Ho R, Cheung CK, et al. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies treated with anticancer therapy. World J Gastroenterol. 2016;22(28):6484-6500. [PMC free article: PMC4968128] [PubMed: 27605883]
- 7.
- Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. [PMC free article: PMC5987259] [PubMed: 26566064]
- 8.
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221-244 e223. [PubMed: 25447852]
- 9.
- Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297-1309. [PMC free article: PMC5501983] [PubMed: 28219691]
- 10.
- Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31(22):2765-2772. [PubMed: 23775967]
- 11.
- Lau GK, Yiu HH, Fong DY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125(6):1742-1749. [PubMed: 14724827]
- 12.
- Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology. 2008;47(3):844-853. [PubMed: 18302293]
- 13.
- Kim SS, Cheong JY, Cho SW. Current nucleos(t)ide analogue therapy for chronic hepatitis B. Gut Liver. 2011;5(3):278-287. [PMC free article: PMC3166666] [PubMed: 21927654]
- 14.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 15.
- Agree Next Steps C. The AGREE II Instrument. [Hamilton, ON]: AGREE Enterprise; 2017: https://www
.agreetrust .org/wp-content/uploads /2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf. Accessed 2022 Aug 24. - 16.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 17.
- Toka B, Koksal AS, Eminler AT, Tozlu M, Uslan MI, Parlak E. Comparison of tenofovir disoproxil fumarate and entecavir in the prophylaxis of HBV reactivation. Dig Dis Sci. 2021;66(7):2417-2426. [PubMed: 32729014]
- 18.
- Buti M, Manzano ML, Morillas RM, et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: the Preblin study. PLoS One. 2017;12(9):e0184550. [PMC free article: PMC5595327] [PubMed: 28898281]
- 19.
- Inada K, Kaneko S, Kurosaki M, et al. Tenofovir alafenamide for prevention and treatment of hepatitis B virus reactivation and de novo hepatitis. JGH Open. 2021;5(9):1085-1091. [PMC free article: PMC8454476] [PubMed: 34584979]
- 20.
- Lee IC, Chao Y, Li CP, et al. Risk of renal events during tenofovir disoproxil fumarate and entecavir antiviral prophylaxis in HBsAg-positive cancer patients undergoing chemotherapy. J Viral Hepatitis. 2018;25(12):1599-1607. [PubMed: 30125436]
- 21.
- Lubel JS, Strasser SI, Thompson AJ, et al. Australian consensus recommendations for the management of hepatitis B. Med J Aust. 2022;216(9):478-486. [PubMed: 35249220]
- 22.
- Christopeit M, Schmidt-Hieber M, Sprute R, et al. Prophylaxis, diagnosis and therapy of infections in patients undergoing high-dose chemotherapy and autologous haematopoietic stem cell transplantation. 2020 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann Hematol. 2021;100(2):321-336. [PMC free article: PMC7572248] [PubMed: 33079221]
- 23.
- Ferraz ML, Strauss E, Perez RM, et al. Brazilian Society of Hepatology and Brazilian Society of Infectious Diseases guidelines for the diagnosis and treatment of hepatitis B. Braz J Infect Dis. 2020;24(5):434-451. [PMC free article: PMC9392086] [PubMed: 32926839]
- 24.
- Doyle J, Raggatt M, Slavin M, et al. Hepatitis B management during immunosuppression for haematological and solid organ malignancies: an Australian consensus statement. Med J Aust. 2019;210(10):462-468. [PubMed: 31104328]
- 25.
- Arora A, Anand AC, Kumar A, et al. INASL guidelines on management of hepatitis B virus infection in patients receiving chemotherapy, biologicals, immunosupressants, or corticosteroids. J Clin Exp Hepatol. 2018;8(4):403-431. [PMC free article: PMC6286881] [PubMed: 30568345]
- 26.
- Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36(30):3043-3054. [PubMed: 30179565]
- 27.
- Coffin CS, Fung SK, Alvarez F, et al. Management of hepatitis B virus infection: 2018 guidelines from the Canadian Association for the Study of Liver Disease and Association of Medical Microbiology and Infectious Disease Canada. Can Liver J. 2018;1(4):156-217. [PMC free article: PMC9202759] [PubMed: 35992619]
- 28.
- Sarmati L, Andreoni M, Antonelli G, et al. Recommendations for screening, monitoring, prevention, prophylaxis and therapy of hepatitis B virus reactivation in patients with haematologic malignancies and patients who underwent haematologic stem cell transplantation-a position paper. Clin Microbiol Infect. 2017;23(12):935-940. [PubMed: 28668466]
- 29.
- Tsai YF, Hsu CM, Hsiao HH. Management of hepatitis B virus reactivation in malignant lymphoma prior to immunosuppressive treatment. J Pers Med. 2021;11(4). [PMC free article: PMC8066124] [PubMed: 33918206]
- 30.
- ASCO guidelines methodology manual. Alexandria (VA): American Society of Clinical Oncology; 2019: https://www
.asco.org/sites/new-www .asco.org /files/content-files /practice-and-guidelines /documents/2019-Guidelines-Methodology-Manual.pdf. Accessed 2022 Jul 26.
Appendix 1. Selection of Included Studies
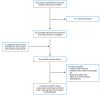
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Note that this appendix has not been copy-edited.
Table 2
Characteristics of Included Primary Clinical Studies.
Table 3
Characteristics of Included Guidelines.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 4
Strengths and Limitations of Clinical Studies Using the Downs and Black Checklist.
Table 5
Strengths and Limitations of Guidelines Using AGREE II.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 6
Summary of Findings by Outcome — HBV Reactivation.
Table 7
Summary of Findings by Outcome — Side Effects.
Table 8
Summary of Recommendations in Included Guidelines.
Appendix 5. References of Potential Interest
- 1.
- Westin J, Aleman S, Castedal M, et al. Management of hepatitis B virus infection, updated Swedish guidelines. Infect Dis. 2020;52(1):1-22. [PubMed: 31613181]
- 2.
- Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan Society of Hepatology guidelines for the management of hepatitis B virus infection: 2019 update. Hepatol Res. 2020;50(8):892-923. [PubMed: 32343469]
Guidelines and Recommendations with Unclear Methodology
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca
- Key Messages
- Context and Policy Issues
- Research Questions
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Antiviral Prophylaxis With Tenofovir for Patients With History of Hepatitis B Re...Antiviral Prophylaxis With Tenofovir for Patients With History of Hepatitis B Receiving Oncology Drug Treatment
Your browsing activity is empty.
Activity recording is turned off.
See more...
