NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lurasidone is a second generation (atypical) antipsychotic agent that is used in the treatment of schizophrenia and bipolar depression. Lurasidone is associated with a low rate of serum aminotransferase elevations during therapy but has not been linked to instances of clinically apparent acute liver injury.
Background
Lurasidone (loo ras' i done) is a second generation antipsychotic agent which appears to act as a dopamine type 2 (D2) and serotonin (5-HT)-2A receptor antagonist in a manner similar to risperidone. Several randomized controlled trials have shown that lurasidone improves symptoms of schizophrenia and it was approved for this indication in the United States in 2010. Indications were subsequently extended to depressive episodes associated with bipolar disorder 2018. Lurasidone is available as tablets of 20, 40, 60, 80 and 120 mg generically and under the brand name Latuda. The recommended starting dose is 20 or 40 mg daily with gradual increase to a maintenance dose of 40 to 120 mg daily, the optimal dose varying by indication, concurrent medication use, renal or hepatic dysfunction and tolerance. Common side effects include somnolence, fatigue, restlessness (akathisia), anxiety, headache, dizziness, constipation, increased appetite, weight gain, orthostatic hypotension and nasopharyngitis. Rare, but potential severe adverse reactions (mentioned in most antipsychotic and antidepressant product labels) include tardive dyskinesia, major neurologic events, neuroleptic malignant syndrome, orthostatic hypotension, seizures, neutropenia, elevations in serum prolactin levels, and suicidal thoughts and behaviors. Furthermore, use of antipsychotic medications in elderly patients with dementia-related psychosis has been associated with increased mortality.
Hepatotoxicity
Liver test abnormalities occur in 1% to 3% of patients on long term therapy with lurasidone, but similar rates have been reported with placebo therapy and with comparator agents. The ALT elevations are usually mild, transient and often resolve even without dose modification or drug discontinuation. There have been no published reports of clinically apparent liver injury with symptoms or jaundice attributed to lurasidone therapy.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which lurasidone might cause serum ALT elevations or liver injury is not known. Lurasidone is extensively metabolized by the cytochrome P450 system (CYP 3A4) to its active metabolite and is susceptible to drug-drug interactions with agents that inhibit or induce CYP 3A4.
Outcome and Management
The serum aminotransferase elevations that occur on lurasidone therapy are usually self-limited and often do not require dose modification or discontinuation. No instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been attributed to lurasidone. Cross sensitivity to liver related or other hypersensitivity reactions between lurasidone and structurally related antipsychotic agents (such as iloperidone, paliperidone, risperidone and ziprasidone) have not been demonstrated, but may well occur.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lurasidone – Generic, Latuda®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lurasidone | 367514-87-2 | C28-H36-N4-O2-S |
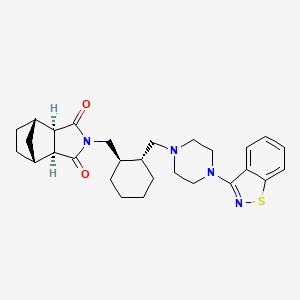
|
| Risperidone | 106266-06-2 | C23-H27-F-N4-O2 |
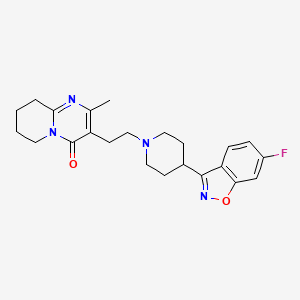
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 June 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Larry D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 507-26.(Review of hepatotoxicity of psychiatric agents does not discuss lurasidone).
- Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, Loebel A. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:829–36. [PubMed: 19497249](Among 180 patients with an acute exacerbation of schizophrenia treated with lurasidone [80 mg daily] or placebo for 6 weeks, adverse events included nausea, vomiting, dyspepsia, constipation, anxiety, restlessness, and somnolence, and one patient stopped therapy early because of ALT and AST elevations, but few details given).
- Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–10. [PubMed: 19321312](Analysis of weight gain in 21 placebo-controlled trials [~3300 patients]; average monthly weight gain in pounds was +0.1 with placebo, +0.8 olanzapine, 0.6 risperidone, -0.3 ziprasidone; a 5% increase in weight occurred after one year in 13% of placebo, 39% haloperidol, 20% ziprasidone, 45% risperidone and 60% olanzapine treated subjects).
- Lurasidone (Latuda) for schizophrenia. Med Lett Drugs Ther. 2011;53:13–4. [PubMed: 21372761](Brief review of efficacy and safety of lurasidone shortly after its approval in the US; common side effects are restlessness [akathisia], extrapyramidal symptoms, agitation, nausea, and somnolence: no mention of ALT elevations or liver injury).
- Potkin SG, Ogasa M, Cucchiaro J, Loebel A. Double-blind comparison of the safety and efficacy of lurasidone and ziprasidone in clinically stable outpatients with schizophrenia or schizoaffective disorder. Schizophr Res. 2011;132:101–7. [PubMed: 21889878](Among 301 patients with schizophrenia treated with lurasidone [120 mg once daily] or ziprasidone [80 mg twice daily], there were no on-treatment laboratory abnormalities).
- Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, Kalali AH, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168:957–67. [PubMed: 21676992](Among 478 patients with acute schizophrenia treated with lurasidone [40 or 120 mg daily] or olanzapine or placebo for 6 weeks, side effects attributed to lurasidone included restlessness, anxiety, agitation, nausea, and somnolence; no mention of ALT elevations or hepatotoxicity).
- Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, Loebel A. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27:165–76. [PubMed: 22395527](Among 629 adults with schizophrenia treated with lurasidone [40 to 120 mg daily] or risperidone [2 to 6 mg daily] for 12 months, “there were no clinically significant changes from baseline to month 12 in liver enzymes”).
- Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74:507–15. [PubMed: 23541189](Among 113 patients enrolled in an open label extension study of lurasidone, the most common adverse events were restlessness, insomnia, somnolence, nausea, headache and weight gain; no mention of ALT elevations or hepatotoxicity).
- Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, Loebel A. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47:670–7. [PubMed: 23421963](Among 496 patients with an acute exacerbation of schizophrenia treated with lurasidone [40, 80 or 120 mg daily] or placebo for 6 weeks, common adverse events were restlessness, headaches, somnolence, nausea, sedation, and weight gain; no mention of ALT elevations or hepatotoxicity).
- Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, Pikalov A, Potkin SG. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145:101–9. [PubMed: 23415311](Among 486 adults with schizophrenia treated with lurasidone [80 or 160 mg daily] or quetiapine [600 mg daily] or placebo for 6 weeks, symptom scores were improved by both agents compared to placebo, while adverse events with lurasidone included restlessness, nausea, dizziness, somnolence, and weight gain, but “no other clinically relevant differences were noted for any other laboratory values” in comparison to the placebo group).
- Drugs for psychiatric disorders. Treat Guidel Med Lett. 2013;11(130):53–64. [PubMed: 23715100](Concise review of safety, efficacy, and role of drugs for psychiatric disorders mentions that lurasidone is a second-generation antipsychotic agent whose adverse side effects include akathisia, nausea, agitation and somnolence; no mention of ALT elevations or hepatotoxicity).
- Loebel A, Cucchiaro J, Silva R, Kroger H, Hsu J, Sarma K, Sachs G. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171:160–8. [PubMed: 24170180](Among 505 patients with major depression and bipolar illness treated with lurasidone [20-60 mg or 80-120 mg daily] or placebo for 6 weeks, symptoms of depression improved with lurasidone therapy, while common side effects were nausea, headache, restlessness, and somnolence and “there were no notable differences in laboratory measures” between treatment groups).
- Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96. [PubMed: 25400109](Extensive systematic review of the literature on the problem of weight gain during therapy with antipsychotic agents mentions that weight gain of 7% or more occurs in 1-14% of patients on lurasidone and averages -1 to +1.3 kg, rates being lower than with most other atypical antipsychotics).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 patients with drug induced liver injury seen over a ten-year period at 8 US medical centers, one case was attributed to olanzapine, but none to lurasidone or other antipsychotic medications).
- Suppes T, Silva R, Cucchiaro J, Mao Y, Targum S, Streicher C, Pikalov A, et al. Lurasidone for the treatment of major depressive disorder with mixed features: A randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2016;173(4):400–7. [PubMed: 26552942](Among 209 adults with major depressive disorder with mixed features treated with lurasidone [20-60 mg daily] or placebo for 6 weeks, symptoms [including mania] improved more with lurasidone, while common adverse events were nausea [6.4%], somnolence [5.5%] and akathisia [3.7%]; no mention of ALT elevations or hepatotoxicity).
- Potkin SG, Kimura T, Guarino J. A 6-week, double-blind, placebo- and haloperidol-controlled, phase II study of lurasidone in patients with acute schizophrenia. Ther Adv Psychopharmacol. 2015;5:322–31. [PMC free article: PMC4722503] [PubMed: 26834965](Among 138 patients with schizophrenia treated with lurasidone [20, 40 or 80 mg daily] or haloperidol and placebo for 6 weeks, there were no differences in psychosis associated symptoms among the different groups and side effects of lurasidone included sedation, dyspnea, nausea and akathisia; no mention of ALT elevations or hepatotoxicity).
- Correll CU, Cucchiaro J, Silva R, Hsu J, Pikalov A, Loebel A. Long-term safety and effectiveness of lurasidone in schizophrenia: a 22-month, open-label extension study. CNS Spectr. 2016;21:393–402. [PubMed: 27048911](Among 251 patients with schizophrenia rolled over into an open-label trial of lurasidone [40 to 120 mg daily] for 6-24 months, there "was no evidence for hepatic toxicity" but were minor changes in ALT and AST levels and 4 subjects [1.7%] "had a markedly abnormal ALT" although none required discontinuation and all except one resolved).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone and lurasidone).
- DelBello MP, Goldman R, Phillips D, Deng L, Cucchiaro J, Loebel A. Efficacy and Safety of Lurasidone in children and adolescents with bipolar I depression: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2017;56:1015–25. [PubMed: 29173735](Among 347 children and adolescents with bipolar depression ages 10 to 17 treated with lurasidone or placebo for 6 weeks, depression symptom scores improved more with lurasidone, and adverse events included nausea [16% vs 6%], somnolence [11% vs 6%], weight gain [7% vs 2%] and insomnia [5% vs 2%]; no mention of ALT elevations or hepatotoxicity).
- Goldman R, Loebel A, Cucchiaro J, Deng L, Findling RL. Efficacy and Safety of lurasidone in adolescents with schizophrenia: a 6-week, randomized placebo-controlled study. J Child Adolesc Psychopharmacol. 2017;27:516–25. [PMC free article: PMC5568017] [PubMed: 28475373](Among 326 adolescents with schizophrenia treated with lurasidone [40 or 80 mg daily] or placebo for 6 weeks, improvements in symptom scores were greater with lurasidone as were side effects of nausea, akathisia, somnolence, and extrapyramidal events; no mention of ALT elevations or hepatotoxicity).
- Pikalov A, Tsai J, Mao Y, Silva R, Cucchiaro J, Loebel A. Long-term use of lurasidone in patients with bipolar disorder: safety and effectiveness over 2 years of treatment. Int J Bipolar Disord. 2017;5:9. [PMC free article: PMC5332323] [PubMed: 28168632](Among 122 patients with bipolar disorder enrolled in short term, controlled trials who were rolled over into an 18-month extension study of lurasidone [mean dose 62 mg daily], adverse events were reported in 43% of patients, including 3% with liver test abnormalities and 1 subject who discontinued therapy early for that reason).
- Higuchi T, Iyo M, Kwon JS, Chou YH, Chen HK, Chen JY, Chen TT, et al. Randomized, double-blind, placebo, and risperidone-controlled study of lurasidone in the treatment of schizophrenia: results of an inconclusive 6-week trial. Asia Pac Psychiatry. 2019;11(3):e12354. [PubMed: 30912222](Among 460 hospitalized Asian patients with schizophrenia treated with lurasidone [40 or 80 mg], risperidone [4 mg] or placebo once daily for 6 weeks, improvement in scores for psychosis symptoms was similar in all 4 groups while adverse event rates were greater with active treatment; no mention of ALT elevations or hepatotoxicity).
- Kato T, Ishigooka J, Miyajima M, Watabe K, Fujimori T, Masuda T, Higuchi T, et al. Double-blind, placebo-controlled study of lurasidone monotherapy for the treatment of bipolar I depression. Psychiatry Clin Neurosci. 2020;74:635–644. [PMC free article: PMC7756283] [PubMed: 32827348](Among 525 Japanese patients with bipolar 1 depression treated with 2 doses of lurasidone or placebo for 6 weeks, improvements in depression symptoms scores were slightly greater with lurasidone [-13.6 and -12.6 vs -10.6] as were adverse events including akathisia [13% and 24% vs 6%] and nausea [6.5% and 11.8% vs 6%], and there was “no evidence of toxicity as measured by laboratory…parameters’).
- Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, et al. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2021;22:373–386. [PubMed: 32892689](Among 246 cases of severe liver injury due to antipsychotic medications identified in a prospective registry of German psychiatric hospitals between 1993 and 2016, 46 arose in 38,349 patients [0.12%] who received clozapine [34 as a single antipsychotic agent]; other commonly implicated agents being olanzapine [n=90 of 54,822: 0.16%], quetiapine [34 of 66,209: 0.05%] and risperidone [27 of 51,683: 0.05%]; two fatal cases occurred in olanzapine-treated patients; low rates were found for ziprasidone [no cases among 3568 patients treated] and aripiprazole [6 cases of 15,988 patients treated: 0.01%]; lurasidone not listed).
- DelBello MP, Tocco M, Pikalov A, Deng L, Goldman R. Tolerability, safety, and effectiveness of two years of treatment with lurasidone in children and adolescents with bipolar depression. J Child Adolesc Psychopharmacol. 2021;31:494–503. [PMC free article: PMC8568779] [PubMed: 34324397](Among 306 adolescents with bipolar depression treated with lurasidone for up to 2 years in open label studies, improvements in symptoms with lurasidone were sustained and adverse events included headache [24%], nausea [16%], and somnolence [10%], and led to drug discontinuation in 10%; no mention of ALT elevations or hepatotoxicity).
- Iyo M, Ishigooka J, Nakamura M, Sakaguchi R, Okamoto K, Mao Y, Tsai J, et al. Efficacy and safety of lurasidone in acutely psychotic patients with schizophrenia: A 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2021;75:227–35. [PMC free article: PMC8361730] [PubMed: 33890388](Among 483 adults with schizophrenia treated with lurasidone or placebo for 6 weeks, improvements in symptoms were greater with lurasidone as were adverse events of akathisia, dizziness, somnolence and abdominal pain, but there were “no clinically meaningful” differences in mean change from baseline in blood chemistry results between the two groups).
- Ishigooka J, Kato T, Miyajima M, Watabe K, Masuda T, Hagi K, Higuchi T. Lurasidone in the long-term treatment of bipolar I depression: a 28-week open label extension study. J Affect Disord. 2021;281:160–167. [PubMed: 33321381](Among 413 adults with bipolar depression who participated in short term controlled trials of lurasidone and were then enrolled in a 28 week, open label, flexible dose [20-120 mg daily] extension study, improvement in symptoms continued and adverse events were mild-to-moderate with no hepatic serious adverse events, and there “was no evidence of toxicity as measured by clinical laboratory” test results).
- Miura I, Watabe K, Sakaguchi R, Okamoto K, Maruyama H. Effectiveness of lurasidone 80 mg in patients with schizophrenia: results of an open-label, 12-week extension study. Neuropsychiatr Dis Treat. 2022;18:2627–2637. [PMC free article: PMC9656454] [PubMed: 36387943](Among 289 adults with an acute exacerbation of schizophrenia participating in a 6 week controlled trial of lurasidone vs placebo who were then treated for 12 weeks with either 40 or 80 mg of lurasidone, symptom improvement was greater with the higher dose, but so were adverse event rates [55% vs 46%], although most were mild-to-moderate and transient; no mention of ALT levels or hepatotoxicity).
- Correll CU, Tocco M, Hsu J, Goldman R, Pikalov A. Short-term efficacy and safety of lurasidone versus placebo in antipsychotic-naïve vs. previously treated adolescents with an acute exacerbation of schizophrenia. Eur Psychiatry. 2022;65:1–35. [PMC free article: PMC9058440] [PubMed: 35322769](Among 326 adolescents with schizophrenia treated with lurasidone or placebo for 6 weeks, improvements in symptoms were greater with lurasidone particularly among treatment-naïve subjects, while side effects were also greater including specific symptoms of akathisia and nausea; no mention of ALT elevations or hepatotoxicity).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine but none for lurasidone, risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- Gunther M, Dopheide JA. Antipsychotic safety in liver disease: a narrative review and practical guide for the clinician. J Acad Consult Liaison Psychiatry. 2023;64:73–82. [PubMed: 36180017](Review of the literature on hepatotoxicity of antipsychotic medications and guidance on their use in patients with liver disease characterizes chlorpromazine, clozapine, and olanzapine as having the greatest risk for causing liver injury, quetiapine and risperidone as having moderate risk, haloperidol as having low risk, and paliperidone, aripiprazole, lurasidone, and loxapine as having low risk).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Asenapine.[LiverTox: Clinical and Researc...]Review Asenapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Management of bipolar I depression: clinical utility of lurasidone.[Ther Clin Risk Manag. 2015]Review Management of bipolar I depression: clinical utility of lurasidone.Findlay LJ, El-Mallakh P, El-Mallakh RS. Ther Clin Risk Manag. 2015; 11:75-81. Epub 2015 Jan 8.
- Review Lurasidone: an antipsychotic with antidepressant effects in bipolar depression?[Australas Psychiatry. 2016]Review Lurasidone: an antipsychotic with antidepressant effects in bipolar depression?Keks NA, Hope J, Castle D. Australas Psychiatry. 2016 Jun; 24(3):289-91. Epub 2016 Apr 1.
- Review The development of lurasidone for bipolar depression.[Ann N Y Acad Sci. 2015]Review The development of lurasidone for bipolar depression.Loebel A, Xu J, Hsu J, Cucchiaro J, Pikalov A. Ann N Y Acad Sci. 2015 Nov; 1358:95-104.
- Review Lumateperone.[LiverTox: Clinical and Researc...]Review Lumateperone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Lurasidone - LiverToxLurasidone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...